Introduction:
Triflic anhydride, also known as trifluoromethanesulfonic anhydride, occupies a prominent position in the toolkit of synthetic chemists. Its unique properties and diverse reactivity make it a valuable reagent in organic synthesis. In this comprehensive blog post, we delve into the intricacies of triflic anhydride, exploring its synthesis, characteristics, and wide-ranging applications.
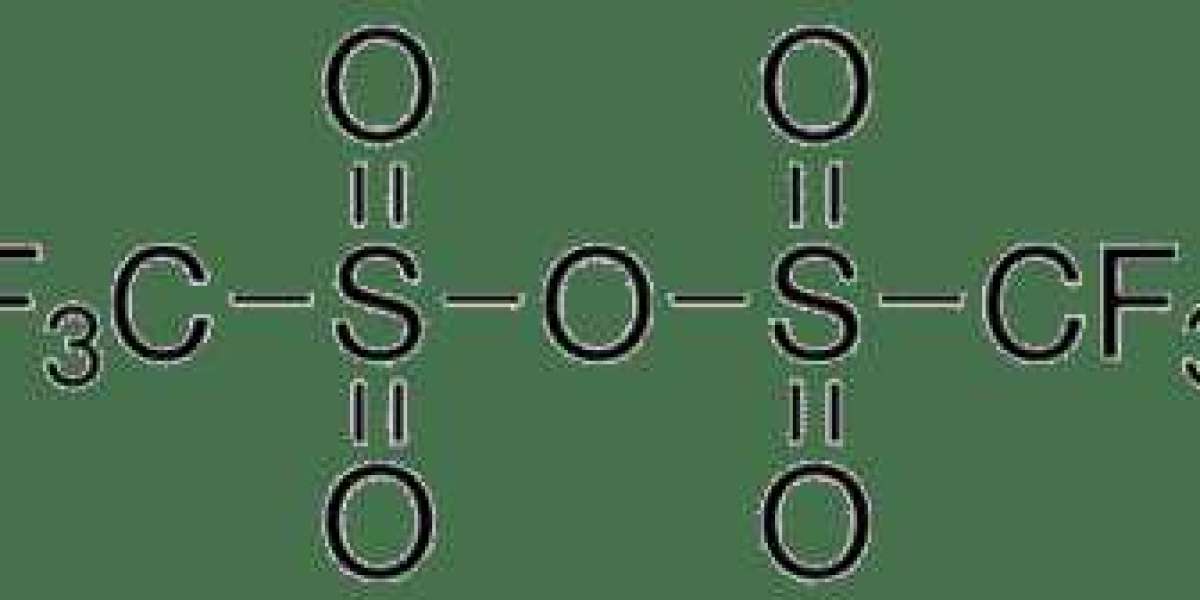
Synthesis and Structure:
Triflic anhydride, with the chemical formula (CF3SO2)2O, is typically synthesized through the reaction of triflic acid (CF3SO3H) with acetic anhydride under controlled conditions. This process yields a cyclic compound containing the trifluoromethanesulfonate (triflate) functional group, known for its exceptional leaving group ability. Triflic anhydride exhibits high reactivity due to the electronegativity of the trifluoromethylsulfonyl group, rendering it a potent electrophile in various organic transformations.
Versatile Applications:
The versatility of triflic anhydride finds expression across a spectrum of synthetic methodologies and applications: