Overview:
- Securing a CE marking is indispensable for medical device manufacturers aiming to introduce their products in the EU market.
- This marking serves as evidence of compliance with rigorous EU regulations and essential standards.
- The CE marking process encompasses a series of critical steps, including identifying relevant directives, conducting thorough gap analyses, establishing a robust Quality Management System (QMS), compiling meticulous technical documentation, and collaborating with Notified Bodies.
What is the CE Marking Process for Medical Devices?
- The CE Marking process is obligatory for products marketed within the European Economic Area (EEA), signifying adherence to EU directives and regulations to ensure safety, efficacy, and quality.
Why is the CE Marking Process Important?
- The CE Marking process holds paramount importance for medical device manufacturers targeting the EU market. It not only facilitates market access but also underscores the commitment to meeting stringent safety, performance, and quality standards, thereby safeguarding public health and promoting seamless trade within the EU.
When is CE Marking Mandatory?
The CE marking process is obligatory for products falling under existing EU regulations that require its application. Certain products must comply with multiple EU requirements simultaneously. Ensuring adherence to all relevant regulations before affixing the CE marking is essential. Affixing the CE marking to products not covered by EU CE Marking specifications or where it is not mandated is prohibited.
Relevant Standards and Regulations
For medical devices, the CE marking process demonstrates compliance with either the Medical Device Regulation (MDR) or the In Vitro Diagnostic Regulation (IVDR), depending on the product category.
The CE Marking Process for medical devices is governed by the MDR and IVDR, which outline essential requirements and conformity assessment procedures:
Regulation (EU) 2017/745 – Medical Devices Regulation (MDR)
This regulation details essential procedures, transition protocols, and clarifications. Medical device Registration EU manufacturers must consistently consult this regulation for accurate information.
Regulation (EU) 2017/746 – In Vitro Diagnostic Regulation (IVDR)
This regulation ensures the smooth operation of the internal market for in vitro diagnostic medical devices, prioritizing patient and user safety while considering the needs of small and medium-sized enterprises.
Directive 2001/83/EC
This directive concerns the marketing authorization of medicinal products intended for human use.
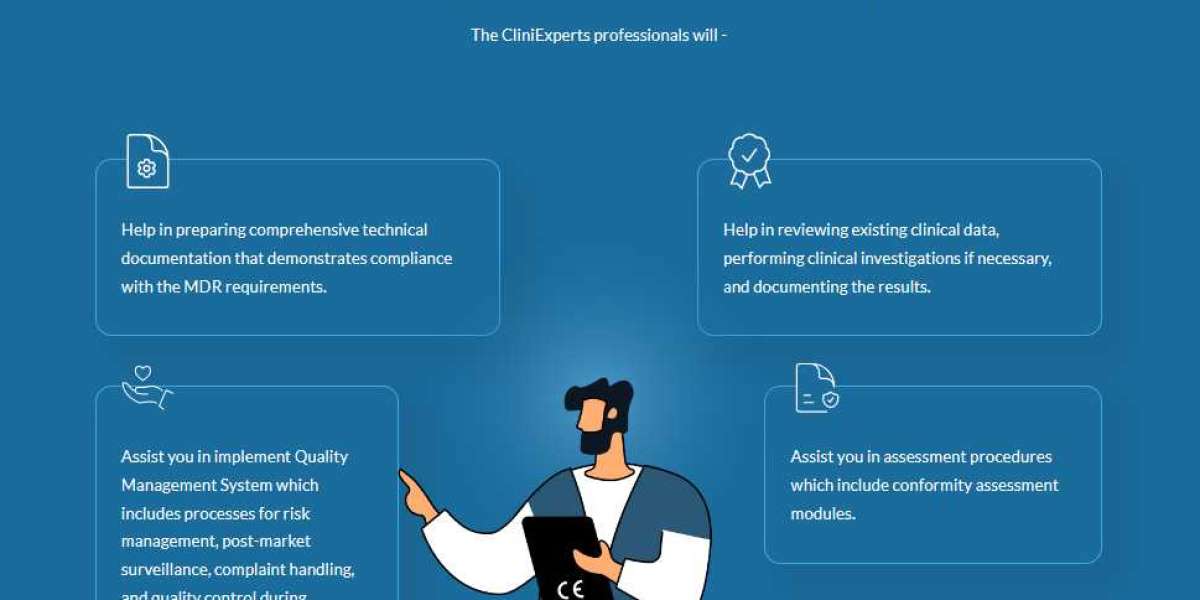
ISO 13485:2016 - Medical devices — Quality management systems — Requirements for regulatory purposes
This standard outlines the criteria for a Quality Management System (QMS) applicable to medical devices.
These regulations are complemented by harmonized standards, offering technical specifications and guidelines for demonstrating compliance with CE Marking Requirements in the EU.
How to Obtain CE Marking for Medical Devices?
Obtaining CE Marking for medical devices involves several key steps:
Identify the applicable directives and regulations based on the product's classification and intended use.
Conduct a gap analysis to evaluate compliance with essential requirements.
Develop and implement a robust Quality Management System (QMS) aligned with relevant harmonized standards.
Compile comprehensive technical documentation, including product specifications, risk assessments, and test reports.
Engage with a Notified Body for conformity assessment, if required.
Affix the CE marking to the product and issue a Declaration of Conformity.
Establish post-market surveillance and vigilance processes.
Fees for Obtaining CE Marking Process
Generally, fees for obtaining the CE Marking Process are not required if a manufacturer conducts the conformity process themselves. However, if they enlist the services of a notified body or if EU specifications necessitate an independent assessment, they must compensate the notified body accordingly.
Duration of Validity for CE Marking
The CE marking does not have a specified validity period. However, maintaining the EU Declaration of Conformity (DoC) is crucial, ensuring it remains current with any changes in legislation, product modifications, or contact details.
CliniExperts, a leading regulatory consulting firm, offers comprehensive services to guide medical device manufacturers through the CE Marking Process. With experienced regulatory experts and a deep understanding of EU regulatory requirements, CliniExperts provides valuable assistance at every stage.
By following best practices, leveraging regulatory expertise, and investing in an efficient CE Marking process strategy, medical device manufacturers can ensure successful market entry within the EU CE Marking framework.
Conclusion
While the CE marking process may seem complex, with the appropriate guidance and expertise, medical device manufacturers can simplify their journey to the European market. By adopting best practices, tapping into the knowledge of regulatory consultants such as CliniExperts, and implementing an effective CE marking process strategy, manufacturers can ensure a smooth and timely entry into the EU market.